
[ASG home page]
[My home page]
[NiF2 Research]
[KJWA's Crypt]
Interstitial Sites
In the nickel fluoride lattice the existance of interstitial nickel and fluorine
ions in the lattice can be considered. When an interstitial ion is introduced
into the crystal the surrounding lattice ions move (relax) in response to the
incorperation of the defect. The extent and nature of this relaxation determins
the magnitude of the defect energy.
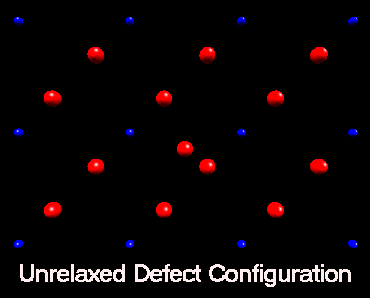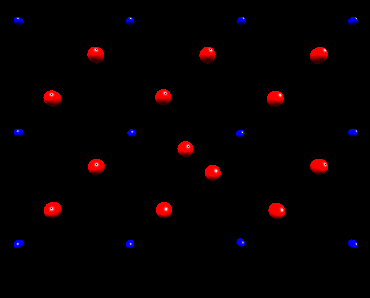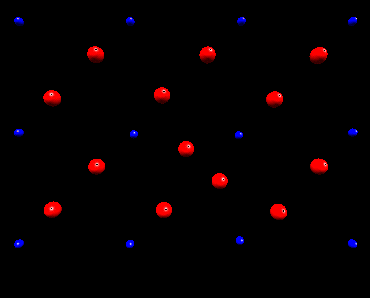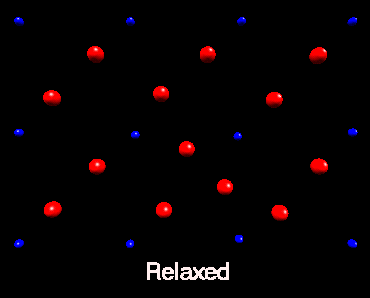
Fluorine Split Interstitial
In nickel fluoride, atomistic simulation predicts that if a fluorine
interstitial is incorperated at an interstitial site the subsequent lattice
relaxation will result in the creation of a split interstitial configoration.
The following animation shows how the lattice responds to the inclusion
of a fluorine interstitial ion. Initially the fluorine ion is placed in
an octahedral interstitial site, subsequently lattice relaxation results in an adjacent
lattice fluorine ion forming a split interstitial. This is predicted
to be the lowest energy configuration. This view is of the basal plane only.
Images created using CASPLOT
(by G.BUSKER)
a utility to visualize the results of CASCADE atomistic simulation calculations.
[ASG home page]
[My home page]
[NiF2 Research]
[KJWA's Crypt]
If you notice any errors
or have any sugestions about this page
then pass them onto the author:
kjwa


